Case Report
Collecting
Duct Renal Cell Carcinoma Found to Involve the Collecting System During
Partial Nephrectomy: A Case Report
Andrew C Harbin1, Brett A Styskel1, Viren Patel2, He Wang2, Daniel D Eun1
1Department of Urology; 2Department of Pathology, Temple University Hospital, 3401 N, Broad Street, Philadelphia, PA 19147, USA.
Abstract
Collecting
duct carcinoma (CDC) is a rare and aggressive form of renal cell carcinoma
(RCC) arising from the principal cells of the collecting duct. One third of cases present with metastatic
disease, but many present in a manner similar to conventional RCC or urothelial
carcinoma (UC). We discuss a case of CDC
which presented as a small mass at the cortico-medullary junction, and was
discovered at robotic partial nephrectomy (RPN) to be grossly involving the
collecting system. A 62-year-old man presented with a small renal mass
suspicious for RCC, which was found on computed tomography (CT) after an
episode of gross hematuria. After
thorough workup, RPN was attempted; however, intraoperatively the mass was
found to be involving the collecting system.
Radical nephroureterectomy was performed, and the pathology report
revealed CDC. CDC is a rare and
aggressive form of RCC. While many cases
are metastatic at diagnosis, most patients present with the incidental finding
of a small renal mass. There are no
reports of a CDC involving the collecting system at RPN after negative
ureteroscopy preoperatively. The
adjuvant therapeutic options for CDC are limited, and long term survival is
poor.
Received: 28 May 2015; Accepted after revision: 17 June 2015; Published: 24 June 2015.
Author
for correspondence: Andrew C Harbin MD, Department of Urology, Temple University
Hospital, 3401 N, Broad Street, Philadelphia, PA 19147, USA. Email: [email protected]
How
to cite: Harbin AC, Styskel BA, Patel V, Wang H, Eun
DD. Collecting Duct Renal Cell Carcinoma Found to Involve the Collecting System
During Partial Nephrectomy: A Case Report. Journal of Kidney Cancer and VHL
2015;2(3):134-139. Doi: http://dx.doi.org/10.15586/jkcvhl.2015.37
Introduction
Collecting duct carcinoma (CDC), also
known as Bellini duct carcinoma, is a rare form of renal cell carcinoma (RCC)
that arises from cells of the collecting duct of the kidney. Although it represents less than one percent
of all RCC cases (1), CDC is particularly aggressive, and up to 32% of cases
may be metastatic at diagnosis (2).
Typical presentation is similar to that of clear cell RCC (1, 3, 4),
though symptoms from metastatic disease or paraneoplastic syndromes at
presentation have been described (5-7).
We discuss a case of CDC that presented
as a centrally located renal mass, not visible on ureterscopy but eventually
found to be grossly invading the collecting system at the time of partial
nephrectomy. Included are pathologic
images and a review of the literature.
Ethics approval
The following review of clinical data
was performed after proper institutional review board approval.
Case Report
We present a 62-year-old man with a
history of hypertension and obesity who developed gross hematuria after a fall
from his bicycle. When the hematuria persisted, magnetic resonance imaging
(MRI) and computed tomography (CT) were performed, revealing a 3.6 x 3.2 x 2.5
cm left upper pole renal mass (Figure 1
A). The mass was mostly endophytic, though still present at the
cortico-medullary junction, so RCC and urothelial carcinoma (UC) were both
potential diagnoses.
Left retrograde pyelogram and ureteroscopy
performed one month prior to definitive surgery were normal, and selective
cytology and brush biopsy were both negative for malignant cells. Chest CT was
negative for metastatic disease. Given this workup, robotic partial nephrectomy
(RPN) was performed for presumed endophytic RCC.
The kidney was fully mobilized and the
main renal artery was clamped, then extirpation was attempted. However, upon
entering the collecting system, the tumor was found to be inside the lumen (Figure 1 B). The immediate concern was
for UC, and the procedure was converted to a nephroureterectomy. The patient recovered from surgery and was
discharged from the hospital on post-operative day two.
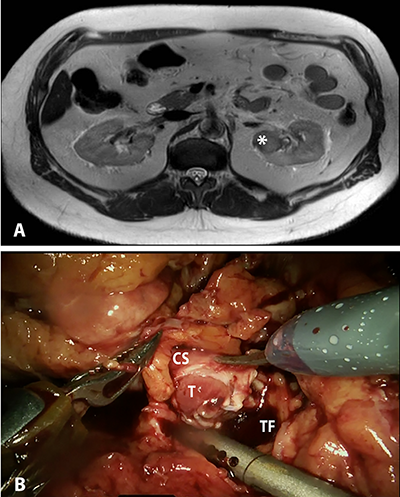
Figure 1. A,
Magnetic resonance imaging (MRI) showing endophytic, posterior upper pole left
renal mass (indicated by *), suspicious for carcinoma. B,
Intraoperative photograph showing grossly invasive mass involving the lumen of
the collecting system. T – Tumor; CS –
collecting system; TF – tumor fossa.
On gross pathologic analysis, a
yellow-tan, circumscribed and lobulated mass measuring 4.2 x 3.5 x 2.7 cm was
found in the cortico-medullary junction of the upper pole. A pale tan tumor thrombus was identified in
the renal pelvis, while no thrombus was identified in the renal vein. On microscopic examination, focal necrosis
and multiple foci of osseous metaplasia were noted (Figure 2). On
immunohistochemistry, tumor cells were positive for PAX8, focally positive for
CA-IX, and largely negative for CK903, p63, and GATA3. These findings are consistent with collecting
duct carcinoma with sarcomatoid differentiation. The tumor was found to be invading the renal
pelvis, renal cortex and perinephric fat; the sinus fat and renal vasculature
were uninvolved. Thirty-five lymph nodes
were removed, and seven were found to contain metastatic cancer.
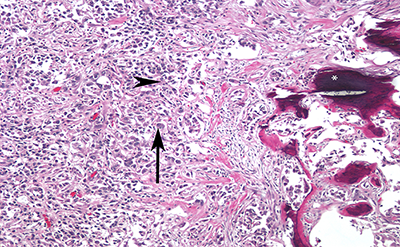
Figure 2. High
magnification photomicrograph showing highly infiltrative carcinoma with
tubular structure, embedded in desmoplastic stroma. The tumor cells have eosinophilic cytoplasm
(arrowhead), the nuclei are large and pleomorphic, with prominent nucleoli
(arrow) and coarse chromatin. Also seen
is evidence of ossification (*).
As the patient presently has no signs
of metastatic disease, he will be followed closely with imaging. Should he suffer from recurrence, he will
likely undergo chemotherapy or combination immunotherapy with targeted therapy.
Discussion
We present a rare case of a CDC that
progressed from noninvasive to collecting system invasion within one
month. CDC is a form of RCC known for
its aggressive nature, and is still poorly understood due to its rarity. It is a malignant epithelial tumor that is
derived from the principal cells of the collecting duct of Bellini, which is
part of the renal collecting system.
This is in contrast to the majority of RCCs, which are derived from the
cells of the proximal convoluted tubules of the nephron. This is also distinct from urothelial
carcinoma (UC), which arises from transitional epithelium of the bladder, renal
pelvis and ureter. It accounts for
<1% of all renal malignancies, and can affect ages 13-83-years old with a
mean age of 55 years. It has a male to
female ratio of 2:1 (8); 63.3% of patients are white, 27.5% are African
American, and 9.2% are other races, according to a large retrospective study by
Pepek et al. (9).
Potential symptoms at presentation
include abdominal pain, hematuria, weight loss or flank mass; or patients may
be asymptomatic (3, 4, 8). Case reports
have described CDC associated with deep vein thrombosis, extensive coagulative
necrosis, syndrome of inappropriate antidiuretic hormone secretion (SIADH) or
leukocytosis secondary to increased granulocyte-colony stimulating factor
(G-CSF) production; however these cases are
atypical (5, 6). About one out of every
three patients has metastases on presentation, and metastases to bone
frequently are osteoblastic (8). Our
patient presented with hematuria but had no signs of metastasis. CDC is a pathologic diagnosis. The diagnosis is made if 1, at least some of
the lesion involves the medullary region; 2, there is a predominant formation
of tubules; 3, a desmoplastic stromal reaction is be present; 4, cytologic
features are high grade; 5, growth pattern is infiltrative; and 6, there is an
absence of other typical RCC subtypes or UC (8). Differentiation between CDC and UC can be
challenging, but the addition of GATA3 to the immunohistochemical profile of
p63 and PAX8 can help discriminate (10).
Moreover, a recent study was able to detect distinct genetic differences
between CDC and UC, concluding that CDC indeed has a distinct genetic pattern
compared to UC. This study observed that
CDC was associated with chromosomal DNA losses at 8p, 16p, 1p and 9p and gains
in 13q, while UC was associated with loss at 9q, 13q, 8q and gains at 8p (11).
While a pathologic diagnosis is
necessary for CDC, several studies have focused on imaging techniques that may
help lead to early diagnosis. A study
pooling 18 cases of proven CDC documented recurring CT findings. The mean longest diameter of the tumor was
6.9 cm and tumors were frequently solid, with a medullary location, weak or
heterogeneous enhancement and infiltrative growth. Vascular invasion only occurred in 28% of
cases (12). Unfortunately, these CT
findings are nonspecific and cannot differentiate CDC from RCC. In our case, UC was suspected given the
location of the mass on imaging.
However, since ureteroscopy and brush biopsy were both negative for
abnormalities, the possibility of RCC involving the collecting system was not
considered likely. It is speculated that
the mass progressed to collecting system involvement rapidly after
ureteroscopy, or that the preoperative imaging inadequately characterized the
mass. There are no descriptions of this
clinical scenario in the literature, though there is one case of CDC diagnosed
by positive cytology (13).The prognosis of CDC is poor as it is
an aggressive disease, and one third of cases are metastatic on presentation
(1). Pepek et al. found that three-year
survival rates for localized, regional and distant disease were 93%, 45% and 6%
respectively (9). Though several
treatment options have been implemented with some success, a standard
chemotherapy regimen is not yet established due to the rarity of CDC. Recently, Dason et al. conducted a systematic
review of the management of CDC (14).
The authors were able to identify three relevant studies, and concluded
that a gemcitabine-cisplatin or gemcitabine-carboplatin regimen offered the
best response. Oudard et al. conducted a prospective
multicenter phase II study evaluating gemcitabine-cisplatin/carboplatin in 23
patients with CDC. The authors reported
a 26% partial/complete response rate (1 complete response), while another 10
patients (44%) experienced disease stabilization, and 7 (30%) had disease
progression (15). Immunotherapy, in the
form of interferon (IFN) and interleukin-2 (IL-2), has been studied and found
to be ineffective (2, 16). In a large
retrospective study of CDC patients from four Japanese institutions, there was
no response 34 patients who received IFN or IL-2 (2). Targeted therapy has also been
evaluated in several very small trials.
Procopio et al. (17) conducted a retrospective study of seven patients
receiving targeted therapy, and identified two patients who lived for 49 months
(sorafenib then sunitinib) and 19 months (temsiroliumus, then sunitinib). Though several other smaller studies exist
(18-20), there is simply not enough data to provide a definitive answer on the
efficacy of targeted therapy in CDC. ConclusionWe present a case of CDC that
progressed to collecting system invasion in a short period of time and required
radical nephrectomy. CDC is an
aggressive form of RCC, associated with low 3-year survival and poor response
to adjuvant therapy. While CDC is rare,
it should be considered in those cases in which the clinical data seem
incongruent with the imaging findings.
Any surgeon attempting a partial nephrectomy should be aware of the
possibility of surprise collecting system involvement, and should be prepared
to revise the surgical plan as necessary.References1. Eble JN, Sauter G, Epstein JI,
Sesterhenn IA. Pathology and Genetics of Tumours of the Urinary System and Male
Genital Organs. World Health Organization Classification of Tumors; Feb 2004, IARC
Press, Lyon.2. Tokuda N, Naito S, Matsuzaki O, Nagashima
Y, Ozono S, Igarashi T. Japanese Society of Renal Cancer. Collecting duct
(Bellini duct) renal cell carcinoma: a nationwide survey in Japan. J Urol. 2006;176(1):40-3. http://dx.doi.org/10.1016/S0022-5347(06)00502-73. Kwon KA et al. Clinical features and
treatment of collecting duct carcinoma of the kidney from the korean cancer
study group genitourinary and gynecology cancer committee. Cancer Res Treat.
2014;46(2):141-7. http://dx.doi.org/10.4143/crt.2014.46.2.1414. Gupta R et al. Carcinoma of the
collecting ducts of Bellini and renal medullary carcinoma: clinicopathologic
analysis of 52 cases of rare aggressive subtypes of renal cell carcinoma with a
focus on their interrelationship. Am J Surg Pathol. 2012;36(9):1265-78. http://dx.doi.org/10.1097/PAS.0b013e31826359545. Xu Q, Cao Q, Liu N, Fang Z, Ye Z,
Peng T. Renal collecting duct carcinoma with extensive coagulative necrosis
mimicking anemic infarct: report of a case and the literature review. Diagn
Pathol. 2013;8:119. http://dx.doi.org/10.1186/1746-1596-8-1196. Yasuda E, Kuwabara H, Shibayama Y.
Collecting duct renal cell carcinoma with the syndrome of inappropriate
antidiuresis: an autopsy case report. Indian J Pathol Microbiol. 2013;56(1):43-6. http://dx.doi.org/10.4103/0377-4929.1161487. Sugiura S, Makiyama K, Nakaigawa N,
Yao M, Kubota Y, Oshiro H. Collecting duct carcinoma producing
granulocyte-colony-stimulating factor (G-CSF). Int J Urol. 2007;14(6):555-7. http://dx.doi.org/10.1111/j.1442-2042.2006.01711.x8. Srigley JR et al. The International
Society of Urological Pathology (ISUP) Vancouver Classification of Renal
Neoplasia. Am J Surg Pathol. 2013;37(10):1469-89. http://dx.doi.org/10.1097/PAS.0b013e318299f2d19. Pepek JM, Johnstone PA, Jani AB.
Influence of demographic factors on outcome of collecting duct carcinoma: a
Surveillance, Epidemiology, and End Results (SEER) database analysis. Clin
Genitourin Cancer. 2009;7(2):E24-7. http://dx.doi.org/10.3816/CGC.2009.n.01710. Gonzalez-Roibon N, Albadine R, Sharma R, Faraj
SF, Illei PB, Argani P, Ertoy D, Allaf ME, Netto GJ. The role of GATA binding
protein 3 in the differential diagnosis of collecting duct and upper tract
urothelial carcinomas. Hum Pathol. 2013;44(12):2651-7. http://dx.doi.org/10.1016/j.humpath.2013.07.006.11. Becker F et al. Collecting duct
carcinomas represent a unique tumor entity based on genetic alterations. PLoS
One. 2013;8(10):e78137. http://dx.doi.org/10.1371/journal.pone.007813712. Yoon SK, Nam KJ, Rha SH, Kim JK,
Cho KS, Kim B, Kim KH, Kim KA. Collecting duct carcinoma of the kidney: CT and
pathologic correlation. Eur J Radiol. 2006;57(3):453-60. http://dx.doi.org/10.1016/j.ejrad.2005.09.00913. Mimura A, Sakuma T, Furuta M,
Tanigawa N, Takamizu R, Kawano K. Sarcomatoid collecting duct carcinoma of
kidney diagnosed with urine and renal pelvic lavage cytology. Diagn Cytopathol.
2010;38(8):603-6.14. Dason S, Allard C, Sheridan-Jonah
A, Gill J, Jamshaid H, Aziz T, Kajal B, Kapoor A. Management of renal collecting duct carcinoma: a
systematic review and the McMaster experience. Curr
Oncol. 2013;20(3):e223-32. http://dx.doi.org/ 10.3747/co.20.1230.15. Oudard S et al. Prospective
multicenter phase II study of gemcitabine plus platinum salt for metastatic
collecting duct carcinoma: results of a GETUG (Groupe d'Etudes des Tumeurs
Uro-Genitales) study. J Urol. 2007;177(5):1698-702. http://dx.doi.org/10.1016/j.juro.2007.01.06316. Motzer RJ, Bacik J, Mariani T,
Russo P, Mazumdar M, Reuter V. Treatment outcome and survival associated with
metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20(9):2376-81. http://dx.doi.org/10.1200/JCO.2002.11.12317. Procopio G, Verzoni E, Iacovelli R,
Colecchia M, Torelli T, Mariani L. Is there a role for targeted therapies in
the collecting ducts of Bellini carcinoma? Efficacy data from a retrospective
analysis of 7 cases. Clin Exp Nephrol. 2012;16(3):464-7. http://dx.doi.org/10.1007/s10157-012-0589-318. Miyake H, Haraguchi T, Takenaka A,
Fujisawa M. Metastatic collecting duct carcinoma of the kidney responded to
sunitinib. Int J Clin Oncol. 2011;16(2):153-5. http://dx.doi.org/10.1007/s10147-010-0116-z19. Staehler M, Haseke N, Schoppler G,
Stadler T, Karl A, Siebels M, Ihrler S, Stief CG. Carcinoma of the collecting
ducts of Bellini of the kidney: adjuvant chemotherapy followed by multikinase-inhibition
with sunitinib. Eur J Med Res. 2008;13(11):531-5.20. Ansari J, Fatima A, Chaudhri S,
Bhatt RI, Wallace M, James ND. Sorafenib induces therapeutic response in a
patient with metastatic collecting duct carcinoma of kidney. Onkologie. 2009;32(1-2):44-6. http://dx.doi.org/10.1159/000183736